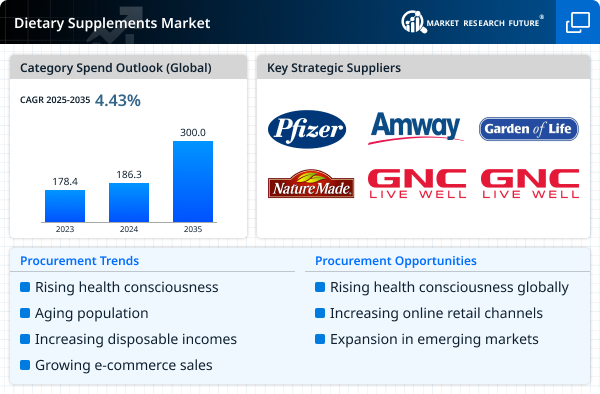
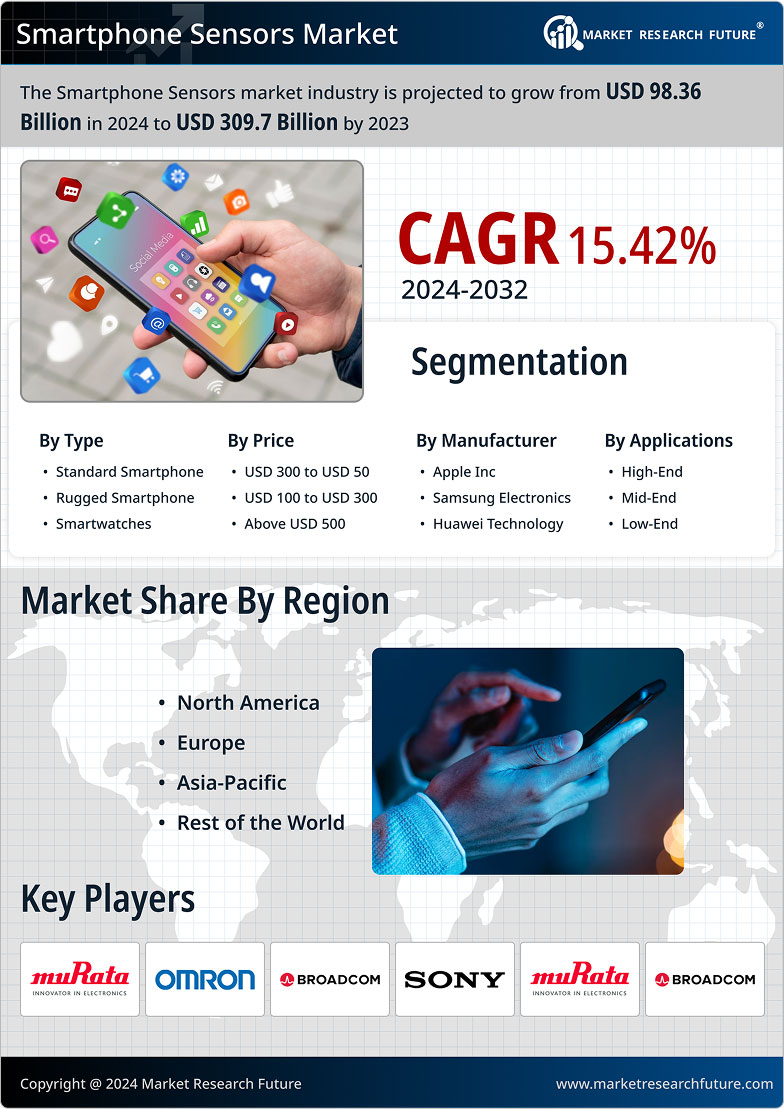
Market Summary
Research Methodology on Clinical Trial Management System Market
The research methodology applied in this study is an in-depth exploration of the global Clinical Trial Management System (CTMS) Market. The research report offers an extensive analysis of the market, encompassing Porter’s Five Forces analysis, PESTEL analysis, value chain analysis, and SWOT analysis. In addition, the report examines the market revenue and volume, drivers, trends, challenges, and opportunities.
Primary Research
The primary research includes in-depth interviews and discussions with technology vendors and industry experts.
Secondary Research
For conducting the research study, secondary research sources are used such as industry journals, white papers, market reports, industry magazines, and paid databases. Details such as market size, forecast, categories and segments, market structure, market dynamics, and competitive insights of the study are collected from various internal and external secondary sources.
Forecast Model
The forecast is based on a detailed review of the strong statistical models used for the research study, combined with information and insights elicited from secondary sources and primary research. The forecast model uses factors such as market dynamics and trends, market segments, and development plans of the leading players to provide an accurate measure of the market.
Market Development
Market development involves the positioning market’s size and its share development in different countries. It focuses on the growth of the primary players in the market and the impact of their strategies on the growth of the global CTMS market.
Market Estimation Techniques
The research uses several techniques for the estimation of the market size. These techniques include bottom-up and top-down approaches. The top-down approach is mostly preferred for the estimation of the global size of the market. In this approach, the market share of the key players operating in the market is used to arrive at an estimation of the total size of the market. The bottom-up approach is extensively used for the estimation of the number of primary players operating in the market.
Data Triangulation
To validate the study findings, market information is triangulated with different techniques such as investment analysis, and the use of various financial synthesis techniques. Thus, the data collected from several sources is triangulated to arrive at an accurate estimate of the global Clinical Trial Management System market.
Scope of the Report
This report offers an in-depth analysis of the global CTMS market. The scope of the report includes an overview of the market and estimates for the period 2023–2030. It provides a detailed assessment of all the key segments in the market and provides market value, drivers, trends, challenges, and opportunities. The report also provides a detailed analysis of the market drivers, trends, market dynamics, and competitive landscape of the global market for CTMS.
Market Size & Forecast
| Attribute/Metric | Details |
| Market Size 2023 | USD 1.88 billion |
| Market Size 2024 | USD 2.16 billion |
| Market Size 2032 | USD 6.50 billion |
| Compound Annual Growth Rate (CAGR) | 14.77% (2024-2032) |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2018 & 2020 |
| Forecast Units | Value (USD Billion) |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | Type, Delivery Model, Component, End User, and Region |
| Geographies Covered | North America, Europe, Asia Pacific, and the Rest of the World |
| Countries Covered | The U.S, Canada, Germany, France, the UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
| Key Companies Profiled | Forte Research Systems Inc. (India), EClinForce, Inc. (US), Oracle (US), Medidata Solutions(US), IWeb Technologies (Canada), Bioclinica (US), Parexel International Corporation. (US), Datatrak Int (US), and Veeva Systems (US) |
| Key Market Opportunities | · The rising frequency of long-lasting diseases |
| Key Market Dynamics | · Rapid advancements in CTMS solutions · The low battery life of mobile computers and reimbursement policies |
Major Players
Market Segmentation Tab for Clinical Trial Management System Market
Clinical Trial Management System Type Outlook (USD Billion, 2018-2030)
- Enterprise CTMS
- On-Site CTMS
Clinical Trial Management System Delivery Model Outlook (USD Billion, 2018-2030)
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
Clinical Trial Management System Component Outlook (USD Billion, 2018-2030)
- Software
- Service
- Hardware
Clinical Trial Management System End User Outlook (USD Billion, 2018-2030)
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
Clinical Trial Management System Regional Outlook (USD Billion, 2018-2030)
-
North America Outlook (USD Billion, 2018-2030)
-
North America Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
North America Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
North America Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
North America Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- US Outlook (USD Billion, 2018-2030)
-
US Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
US Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
US Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
US Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Canada Outlook (USD Billion, 2018-2030)
-
Canada Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Canada Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Canada Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Canada Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
-
North America Clinical Trial Management System by Type
- Europe Outlook (USD Billion, 2018-2030)
-
Europe Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Europe Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Europe Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Europe Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Germany Outlook (USD Billion, 2018-2030)
-
Germany Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Germany Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Germany Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Germany Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- France Outlook (USD Billion, 2018-2030)
-
France Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
France Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
France Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
France Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- UK Outlook (USD Billion, 2018-2030)
-
UK Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
UK Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
UK Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
UK Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Italy Outlook (USD Billion, 2018-2030)
-
Italy Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Italy Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Italy Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Italy Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Spain Outlook (USD Billion, 2018-2030)
-
Spain Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Spain Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Spain Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Spain Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Rest Of Europe Outlook (USD Billion, 2018-2030)
-
Rest Of Europe Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Rest of Europe Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Rest of Europe Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Rest of Europe Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
-
Europe Clinical Trial Management System by Type
- Asia-Pacific Outlook (USD Billion, 2018-2030)
-
Asia-Pacific Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Asia-Pacific Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Asia-Pacific Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Asia-Pacific Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- China Outlook (USD Billion, 2018-2030)
-
China Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
China Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
China Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
China Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Japan Outlook (USD Billion, 2018-2030)
-
Japan Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Japan Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Japan Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Japan Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- India Outlook (USD Billion, 2018-2030)
-
India Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
India Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
India Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
India Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Australia Outlook (USD Billion, 2018-2030)
-
Australia Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Australia Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Australia Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Australia Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- South Korea Outlook (USD Billion, 2018-2030)
-
South Korea Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
South Korea Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
South Korea Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
South Korea Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Rest of Asia-Pacific Outlook (USD Billion, 2018-2030)
-
Rest of Asia-Pacific Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Rest of Asia-Pacific Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Rest of Asia-Pacific Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Rest of Asia-Pacific Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
-
Asia-Pacific Clinical Trial Management System by Type
- Rest of the World Outlook (USD Billion, 2018-2030)
-
Rest of the World Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Rest of the World Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Rest of the World Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Rest of the World Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Middle East Outlook (USD Billion, 2018-2030)
-
Middle East Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Middle East Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Middle East Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Middle East Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Africa Outlook (USD Billion, 2018-2030)
-
Africa Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Africa Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Africa Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Africa Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
- Latin America Outlook (USD Billion, 2018-2030)
-
Latin America Clinical Trial Management System by Type
- Enterprise CTMS
- On-Site CTMS
-
Latin America Clinical Trial Management System by Delivery Model
- Web-Based (Hosted CTMS)
- Licensed Enterprise
- Cloud-Based CTMS (SaaS)
-
Latin America Clinical Trial Management System by Component
- Software
- Service
- Hardware
-
Latin America Clinical Trial Management System by End User
- Pharmaceutical And Biopharmaceutical Companies
- Medical Device Manufacturers
- Contract Research Organizations
- Others
-
Rest of the World Clinical Trial Management System by Type

Leave a Comment